建立細胞模仿平台:由下而上策略逐步重建仿生細胞之不對稱分裂
Towards artificial cell differentiation: bottom-up approach for reconstituting asymmetric division
計畫主持人:臺大分生所-黃筱鈞、中研院化學所-涂熊林
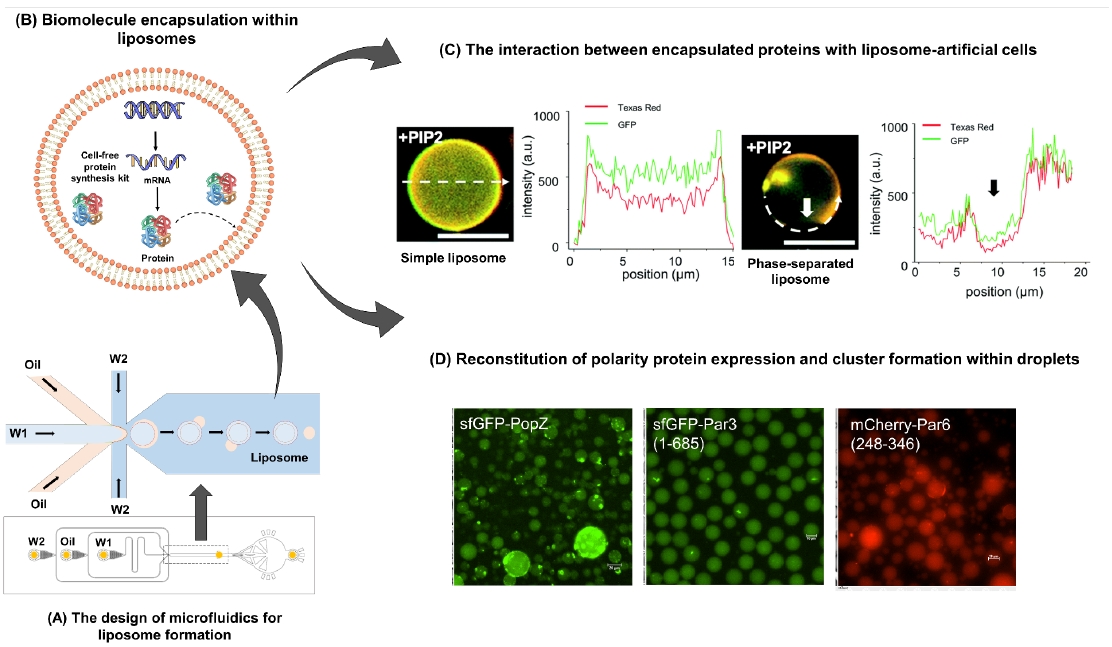
Biological cells are highly delicate systems and contain many biomolecules, reactions, and phonotypes. Creating an ideal model for understanding it remains a substantial challenge. In this project, we demonstrated a robust microfluidic strategy to rapidly generate size-tunable liposomes with good uniformity and custom lipid compositions. Particularly, these liposomes possess the ability to encapsulate various biomolecules, including proteins, DNA plasmid and IVTT reagents. The results showed enclosed proteins can interact with PIP2 lipid on the liposomal membranes. Moreover, this system can generate phase-separated liposomes to recaptulate protein-lipid interactions, indicating the versatility of this approach. Size-tunable water-in-oil droplets have also been generated with a slightly modified microfluidic strategy. The oligomeric polarity modules upstream in the asymmetric cell division pathway, such as bacterial PopZ and mammalian Par-3/Par-6, have been successfully encapsulated. Under current bacterial expression systems (i.e. T7 polymerase + IVTT reactions and E. Coli-based cell extracts), we have reconstituted PopZ clusters as visible foci inside droplets, currently sized at 10-20μm in diameter. Par-3 and Par-6 clusters were scored in a fraction of droplets. Based on biochemical results, robust foci could be anticipated once mammalian expression systems are adopted. In sum, we have established a quantitative platform for invesitgating molecule interaction and symmetry breaking underlying asymmetric cell división. We envision results from this project could serve as a first step towards artificial cell differentiation.
了解細胞的調控網絡是生物學的重要課題。由於包含了許多成分、反應和表型,因此如何創建理想之細胞模型來探討相關研究一直相當具挑戰性。透過此計畫,我們開發微流晶片並建立一個可快速生成尺寸可調與具良好均一性和客製脂質的微脂體之方法,並藉此研究細胞之分子作用和非對稱分裂。這些微脂體可封裝各式分子,包含蛋白質、DNA質體和IVTT試劑,並可以重現蛋白質與微脂體上PIP2脂質的交互作用。此外,我們也通過此方法生成相分離的微脂體,進一步表明此方法的功能性。另計畫中也調整微流晶片,使其可生成尺寸可控的水包油微滴。我們成功地將細菌中的PopZ和哺乳細胞之Par-3/Par-6蛋白質封裝在微滴中。其中上述的蛋白質主要於非對稱分裂途徑扮演重要角色。結果顯示高度聚集之PopZ可於實驗微滴內被觀察到。此外,在部分的微滴中,我們也能觀測到Par-3和Par-6的聚集訊號。整體來說,通過此計畫我們成功建立了一個量化平台,並用於直接重現蛋白質與脂質之作用。我們期望能藉此結果繼續建立更接近細胞之仿生分析平台。