利用全基因體DNA甲基化與轉錄體解析類3號DNA甲基化酶(DNMT3L)對前精原細胞中DNA甲基化之調控
The impact of DNMT3L in the dynamics of genome wide DNA methylation in the prenatal germline
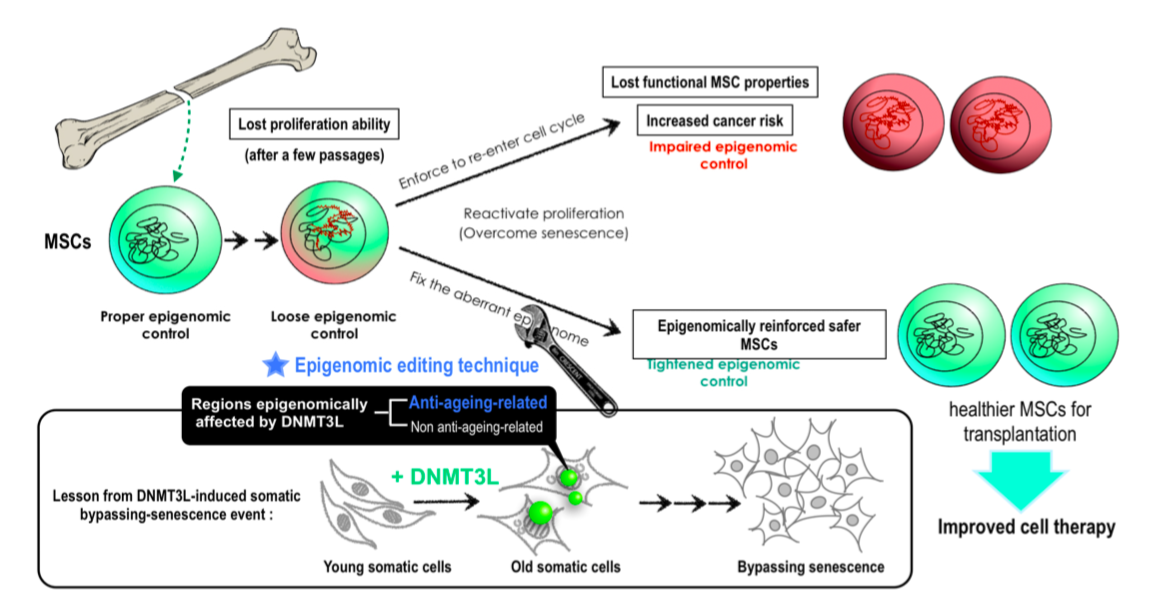
Guiding epigenetic modifiers to the right place at the right time are critical for establishing specific epigenomic signature necessary for establishing and maintaining cell identity and function. DNA methyltransferase 3-like (DNMT3L) being an epigenetic modulating factor, mainly expressed in embryonic stem cells and germ cells, facilitating de novo DNA methylation by partner with DNMT3A and DNMT3B on chromatin region without methylation on histone3 lysine 4 (H3K4). In addition, we observed that ectopic DNMT3L in somatic cells assemble the repressive epigenetic modulators DNMT3L/ DNMT3A/ KAP1/ HDAC1 (histone deacetylase 1)/ SETDB1(H3K9 methyltransferase). We therefore proposed that DNMT3L is one of the top candidates required for guiding the establishment of epigenomic signatures critical for cell fate determination. DNMT3L has mainly been addressed to play roles in embryonic stem cells and in germ line. These include our work in identifying the DNMT3L regulated route of CDK2-PLZF-SALL4A to ERK and AKT cascade that are important to maintain long term male fertility via balancing the quiescence and proliferation of spermatogonia stem cells, and therefore the homeostasis within testes. Outside of germ cell lineage, the expression and function of DNMT3L are very much under debate.
Our current project revealed (for the first time) that the transient DNM3L expression in those primitive cells provide long term effect in somatic mesenchymal lineages that would affect the somatic stem cell property and feasibilities for stem cell mediated regenerative medical applications. The resonation of transient DNMT3L expression in long term cell fate influence has been studied and discussed.
As DNMT3L localized in human chromosome 21, significant elevation of DNMT3L expression in the frontal cortex of Down syndrome patient have been observed and implicated to be contributing to cognitive impairment. The ongoing analysis will further illuminate the mechanism behind how transient DNMT3L expression in pluripotent stem cells affect the transcriptome and cell fate of later differentiated lineages. The outcome of this model would provide a unique angle that may apply for the delayed response from DNMT3L expression to the observation of phenotypes in germ cells, cancer cells, aging cells and neurological lineages.

類 3 號 DNA 甲基化酶(DNA methyltransferase 3-like, DNMT3L)為主要表現於胚胎幹細胞和⽣殖細胞的表觀遺傳因⼦,其雖不具酵素功能,但協同 DNMT3A 與 DNMT3B 催化DNA 甲基化之建⽴。除此之外,本團隊過去發現,將 DNMT3L 短暫表現於⽼化的⼩⿏胚胎纖維母細胞(mouse embryonic fibroblasts, MEFs)中,會召集表觀基因調控蛋⽩形成複合體,加強跳耀⼦靜默機制。因此,我們認為 DNMT3L 是引領表觀基因調控蛋⽩建⽴決定細胞命運的表觀基因組特徵的關鍵因⼦之⼀。
與 DNMT3L 相關之研究多半闡述其在胚胎幹細胞以及⽣殖細胞內扮演的⾓⾊,然⽽,在其他細胞系的了解甚少。本研究即第⼀次指出於胚幹細胞等始基細胞表現之 DNMT3L,其效應會延續⾄間質幹細胞,研究發現Dnmt3l 基因缺失⼩⿏分離出的間質幹細胞,⾃我更新能⼒以及成⾻分化能⼒明顯下降,且轉錄組分析結果顯⽰野⽣型和缺乏 Dnmt3l ⼩⿏來源的間葉幹細胞之間差異表達基因(Differentially expressed genes, DEGs)與⾻頭型態的相關途徑相關 ; ⽽誘導硬⾻分化三天的差異表達基因則顯⽰和微管(microtubule)的動態活性有關。這些基因表現分析闡釋 Dnmt3l 突變⼩⿏來源的間葉幹細胞不正常的基因表達可能與失去幹細胞特性和硬⾻分化能⼒有關。本研究亦討論短暫表現 DNMT3L 卻得以在⽣殖細胞、成體幹細胞、⽼化細胞造成⾧期影響之機制,此研究成果對於不孕症、癌症之成因,與再⽣醫學之應⽤皆提供顯著貢獻。