上皮-間質轉化(EMT)與晝夜節律基因 Period2(PER2)在上皮卵巢癌(OC)之雙向調控
Bi-directional regulation between epithelial-mesenchymal transition (EMT) and circadian gene Period2 (PER2) in epithelial ovarian cancer (OC)
計畫主持人:臺大醫學系-黃韻如、中研院基因體中心-黃雯華
Recent evidence has shown that circadian disruption and clock gene dysregulation promote tumorigenesis and cancer progression. How circadian gene dysregulation contributes to ovarian cancer (OC) progression within the context of molecular heterogeneity remain elusive. We leveraged on the database ‘CSIOVDB’ (http://csiovdb.mc.ntu.edu.tw/CSIOVDB.html) to identify genes and pathways that exhibit gene-expression based molecular subtypes (GEMS)-specific survival differences. Our analysis showed that the circadian gene Period 2 (PER2) was the only core clock gene displaying highly significant differential expression among OC GEMS. PER2 gene expression is significantly downregulated in ovarian tumors compared to normal ovarian surface epithelial tissue and negatively correlated with the OC grade. OC patients with high PER2 level (upper 25%, Q4) had better overall survival compared to the patients with low PER2 level (lower 25%, Q1). These results suggest that PER2 expression may be critical to prevent cancer progression in OSE cells.
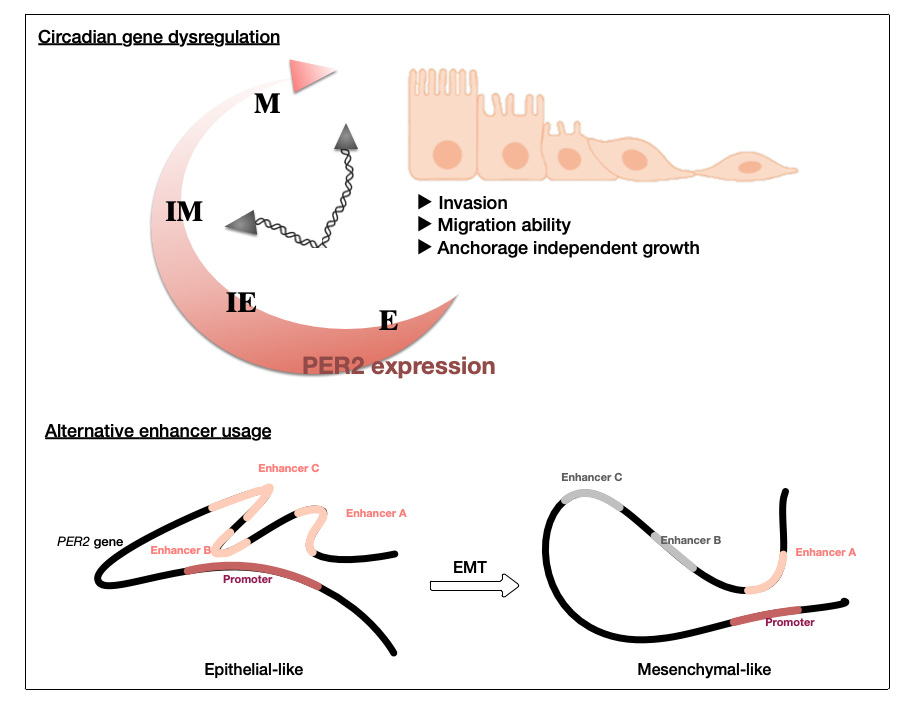
Interestingly, high PER2 expression was significantly associated with better disease-free and overall survival within the Epi-A subgroup but not the other subgroups. Our data thus far indicates that the alternative enhancer usage can control PER2 transcription resulting in EMT in the Epi-A subtype of OC cells. PER2 depletion did not have significant effect on cell proliferation. Instead, tumorigenic potential including anchorage independent growth, invasion and migration ability was significantly elevated upon PER2 down regulation. These findings suggested that PER2 acts as a tumor suppressor in phenotypically normal OSE cells and regulates EMT in the Epi-A subgroup OC cells.
Collectively, our data thus far is pointing to a unique tissue context of PER2 regulation in EMT. This opens a new dimension in how genomic regions similar to the PER2 enhancers might control the execution of EMT via the genome organization. Additional cell-based assays, molecular analysis and mouse model or clinical analysis confirming the key regulatory mechanisms of PER2 within the context of OC are under way. Results from these experiments will be a breakthrough in both circadian biology and cancer research fields.
近年來有多方研究顯示,生理時鐘紊亂和其基因失調會促成腫瘤生成,也和癌症的惡化有關連。然而,針對卵巢癌(OC)細胞因分子異質性惡化的過程,生理時鐘基因失調如何影響其機制目前仍不清楚。我們利用CSIOVDB資料庫(http://csiovdb.mc.ntu.edu.tw/CSIOVDB.html)鑑定,找出卵巢癌在不同亞型中重要的致癌基因與途徑,藉此發現Period2(PER2)是唯一有明顯差異表達的核心生理時鐘基因。卵巢腫瘤中PER2基因的表現量相較正常卵巢表面上皮組織來得低,且其表現量與OC的惡性等級(grade)呈負相關,另外,相較具有低PER2表現量(Q1)的患者,高PER2表現量(Q4)的OC患者有較高的存活率,顯示PER2的基因表達可能對抑制卵巢組織的癌化進程至關重要。
值得令人關注的是,PER2只在Epi-A亞型的細胞中高度表現,並與病人的存活率有顯著正相關。我們分析發現,各亞型內染色質的狀態即有明顯差異,從實驗結果得知,OC選擇性得使用增強子進而調控PER2轉錄,且同時使細胞產生EMT轉型。當抑制OC的PER2表現時,細胞增殖沒有顯著影響;然而,當正常的卵巢細胞的PER2下調後,侵襲和遷移的能力顯著提高,可見細胞失去PER2後有明顯的癌化現象。這些結果皆表明PER2在表型正常的卵巢表皮細胞中,可能具有抑制癌化的功能,並調節Epi-A亞型OC細胞的EMT。
綜合以上結果,我們揭發了PER2在卵巢癌的EMT中扮演重要調控的角色,也為類PER2增強子的基因組區域如何透過基因組交織調控EMT的機制開闢了一個新的維度,這些研究結果將成為晝夜節律生物學和癌症研究領域的一大突破。