以小鼠模型發展思覺失調症的新治療靶點和治療藥物
Identifying new therapeutic targets and a new class of compounds for the treatment of schizophrenia in the mouse models of schizophrenia
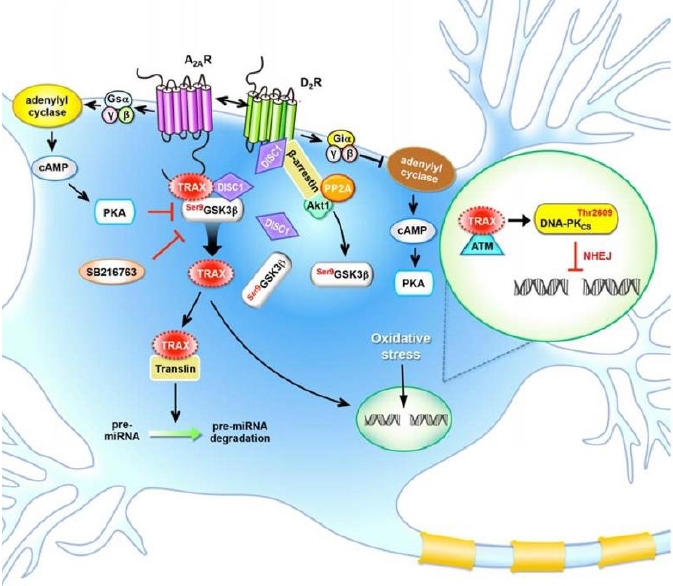
思覺失調症的負性與認知症狀治療迄今仍是未被滿足的醫療需求。過去文獻指出
adenosine 可能參與思覺失調症的症狀與調控機制。為發展治療思覺失調症的新治療靶點
與藥物,本研究計畫於 2019 與 2020 年在台大與中研院創新合作計畫的支持下進行兩線
的研究。第一線研究以 TRAX 基因剔除小鼠為模式,用以探討 A2AR 下游蛋白質 TRAX 與
D2R 分子路徑之互動,以驗證根據過去研究所建立的 TRAX-GSK3β-DISC1 假說(如附圖
所示)。我們的實驗結果發現 TRAX 扮演調節 A2AR 和 D2R 的分子訊息傳遞的角色,降低
腦中 TRAX 的量( knockdown 或 knockout )會使小鼠對多巴胺 D2受體藥物(包括 D2拮
抗劑與甲基安非他命)的刺激較敏感,對於 A2AR 的拮抗劑刺激則較不敏感。另外透過免
疫染色法也發現TRAX、GSK3β與DISC1三者在紋狀體中分布重疊,這些實驗結果支持
TRAX-GSK3β-DISC1假說。第二線研究針對一個新穎的小分子化合物( MF3 )進行研究。
MF3 具有調控腦中 adenosine tone 的效用,有可能可以透過 A2AR-D2R 交互作用來治療
思覺失調症相關症狀。我們的實驗結果發現 MF3 可以顯著抑制甲基安非他命促發之過度
活動(一種思覺失調症的動物模型),但並不影響甲基安非他命所引起的制約場域偏好反
應或成癮效果。本研究成果有助於深入瞭解 A2AR-D2R 交互作用的分子機制,同時也證實
可能可以透過小分子藥物(如 MF3 )來調控 A2AR-D2R 交互作用進而改善思覺失調症相關
症狀。
To date, the negative and cognitive symptoms of schizophrenia remain to be an unmet
medical need. Accumulating evidence revealed that adenosine might be involved in the
pathogenesis of schizophrenia. With supports from the National Taiwan University and
Academia Si nica Innovative Joint Program (2019 2020), we aimed at developing new
therapeutic targets and potential drugs for the treatment of schizophrenia. The goal of
this two year collaborative project is two fold: we first employed TRAX null mice as a
model to de termine the role of TRAX-GSK3β-DISC1 axis in the A2AR-D2R interaction as illustrated in the figure. Our current findings showed that mice had no TRAX were more
sensitive to D2R ligands (i.e., an antagonist and methamphetamine (METH)) and less
sensitive to an A2AR ligand (i.e., an antagonist). The co localization of TRAX and GSK3β
in the striatum revealed by immunofluorescence staining further support our TRAX-
GSK3β-DISC1 hypothesis. Second, we focused on MF3, a small adenosine analogue,
which can regulate a denosine tone in the brain. We hypothesized that, by controlling the
interaction between A2AR and D2R, MF3 may be used to treat schizophrenia. Consistent
with our hypothesis, MF3 significantly suppressed the METH induced hyperlocomotion
(i.e., a classical animal model of schizophrenia), but not the METH induced behavioral
sensitization or rewarding in the conditioned place preference test. Collectively, our
results advance the molecular understanding of the A2AR-D2 interaction and suggest that
modulation of the A2AR-D2 signaling complex by controlling the adenosine tone is a
potential approach to develop new therapeutic treatments for schizophrenia.