研發整合微流道系統之超高解析度顯微鏡進行細胞主纖毛之力學與化學刺激反應研究
Determining mechanical and chemical stimulation responses of primary cilia with an integrated microfluidics-superresolution platform
This project integrates the microfluidics and super-resolution microscopy to study how primary cilia react to shear stress stimulation. Primary cilium is a versatile organelle in mammalian cells, it can sense various kinds of signal and transmit to cells. However, the pathway of primary cilia to inform cells is still unclear and need to be studied by integrating micro-meter scale technique like microfluidic and super-resolution microscopy. In this program, by using our systems, we focus on how the shear stress affect primary cilia, and how primary cilia react to the stimulation. Combining the precise fluidic manipulation of microfluidics and the high accuracy of super-resolution microscopy, we can observe the protein localization inside primary cilia after giving desirable shear stress. Our microfluidic device provided a well-confined cell culture environment with precise flow shear control. Importantly, the in-situ fixation process preserved the ciliary phenotype and allowed for the observation of cilium-specific protein under different flow shears. We characterized individual cilium bending postures under different flow shears by measuring their projection length and alignment angle. In the below figure, for each shear stress condition, ~250–350 cilia were individually imaged (Fig. 1). Under high shear-stress stimulation, over 50% of the primary cilia were highly aligned with the flow direction, indicating uniform flow shear within the device. This result proof the feasibility of providing multiple fluid condition and in-situ fixation in the microfluidic channel. Finally, we used super-resolution microscopy to observe the cilium-related protein profile. To the best of our knowledge, this is the first platform combining a microfluidic device with super-resolution microscopy to enable precise flow stimulation and in-situ cell fixation, allowing detailed observations of the proteins inside the cilia. These features could help us identify which proteins are involved in the mechanosensing of primary cilia.
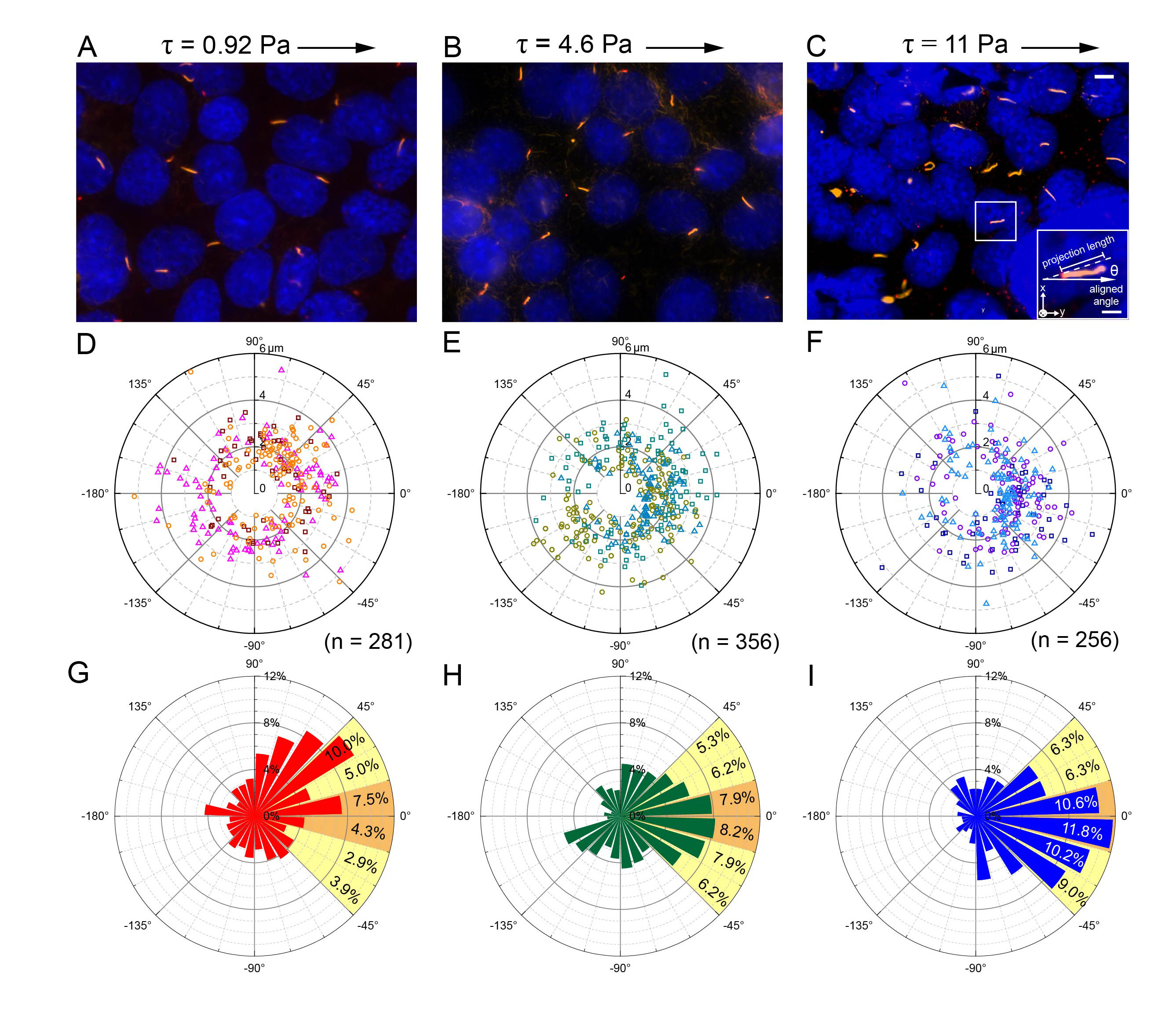
Fig. 1 (A–C) Images of immunostained cilia showing their bending under 3 shear stress levels. (D–F) The distribution profile of individual primary cilia in polar coordinates. Three individual experiments were performed under each condition. Cilia from an individual experiment are shown in the same color. The total numbers of cilia under the different shear stress levels were 281, 356 and 256, respectively. (G–I) Angular distribution of the cilia in polar coordinates. The proportion of each interval between ±45° is also shown next to the bar. The orange and yellow colors represent those highly (±15 degrees) and moderately (±45 degrees) correlated to the direction of the flow stimulation, respectively.
本計畫希望開發整合微流道及超解析顯微鏡之系統觀察哺乳動物細胞中主纖毛受到力學刺激後的變化。主纖毛是一種偵測環境中變化的胞器,研究顯示流場變化、分子濃度、光等外在刺激都能透過主纖毛來傳遞刺激訊號至細胞內。其中偵測物理性的刺激為主纖毛的主要功能,且與許多疾病相關聯。然而,由於主纖毛的尺寸在微米等級,一般研究方法無法給予精準的控制,同時直接觀察主纖毛在刺激下的變化更為困難,目前研究多利用主纖毛的長度、細胞內鈣離子濃度、基因表現來觀測主纖毛的功能。微流道是一種在微米等級下控制流體的技術,具有體積小、價格低廉、低樣本需求量等優點。其中精準的流體控制,為微流道特別適合運用在主纖毛研究上的原因,無論是給予流場刺激、液體的切換,都較傳統的研究方法更加方便。而超解析顯微鏡則可清楚地觀測主纖毛內部的蛋白質的組成、移動或是數量變化,進而得知主纖毛是如何調控及傳輸這些外部刺激到細胞裡。為提供一個能給予適當刺激、在活細胞以及免疫染色下都方便觀察的實驗架構,本研究開發出結合多流速、可拆卸式等優點的微流道系統,以精準的流場控制觀察活細胞下的主纖毛受流場刺激後的變化,同時也能在拆解之後針對想觀察的蛋白質進行免疫染色,最後透過螢光顯微鏡或是超解析顯微鏡來分析刺激之後的結果。在實驗中,我們最佳化細胞培養的參數來產生高密度的主纖毛,也觀測到在活細胞下主纖毛受到流場刺激的變化。透過免疫染色以及觀察大量的主纖毛。最後我們發現主纖毛中的蛋白質分佈會受到流場的刺激而改變,此結果與主纖毛的訊息傳遞功能高度相關。未來期望此系統能與蛋白質譜量測技術整合,進行高通量的刺激同時針對主纖毛特有蛋白質表現進行更深入的研究。

Fig. 1 (A–C) Images of immunostained cilia showing their bending under 3 shear stress levels. (D–F) The distribution profile of individual primary cilia in polar coordinates. Three individual experiments were performed under each condition. Cilia from an individual experiment are shown in the same color. The total numbers of cilia under the different shear stress levels were 281, 356 and 256, respectively. (G–I) Angular distribution of the cilia in polar coordinates. The proportion of each interval between ±45° is also shown next to the bar. The orange and yellow colors represent those highly (±15 degrees) and moderately (±45 degrees) correlated to the direction of the flow stimulation, respectively.