發展針對阿茲海默症中乙形類澱粉蛋白寡聚體之新穎小分子抑制物及核磁影像探針
Development of Novel Small Molecule Inhibitors and MRI Probes Targeting Alzheimer’s Amyloid-β Oligomers
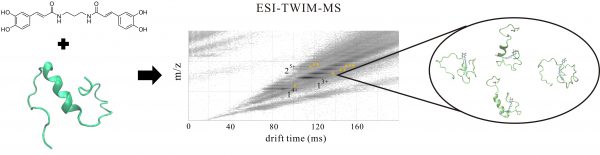
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder affecting tens of millions people. The pathologic hallmarks are extracellular senile plaque composed of amyloid-β (Aβ) fibrils and intracellular neurofibrillary tangles comprising hyperphosphorylated tau proteins. Blocking Aβ self-assembly or disassembling Aβ insoluble plague by small molecules would be potential therapeutic strategies to ameliorate Aβ aggregation-induced neuronal toxicity in AD. In work presented here, we synthesized and examined the effect of a series of new rationally designed bivalent synthetic compounds on Aβ fibrillization by thioflavin T (ThT) assay and by transmission electron microscopy (TEM). One of these compounds, diCA, the divalent amide form of caffeic acid (CA), possessing a propylene diamine linker of ~5.0 Å length that is close to the distance of intermolecular β sheets in Aβ fibrils showed good potency to inhibit Aβ fibrillization and reduce Aβ toxicity in human neuroblastoma and the Aβ transgenic C. elegans. In addition, the specific binding of diCA to Aβ was demonstrated by using electrospray ionization-traveling wave ion mobility-mass spectrometry (ESI-TWIM-MS). Molecular simulation was also conducted to gain structural insights for Aβ and diCA complex. Our results present a new potential for AD therapeutics.
阿茲海默症(AD)是影響數千萬人的漸進性神經變性。病理特徵包含由類澱粉蛋白β(Aβ)纖維組成的細胞外老年斑塊和含高度磷酸化tau蛋白的細胞內神經纖維糾結。阻斷Aβ組裝或透過小分子拆解Aβ不溶性斑塊將是改善AD中Aβ聚集誘導之神經元毒性的潛在治療策略。在這裡,我們合成了一系列新穎符合邏輯性設計的二價合成化合物,並檢視其對Aβ由硫代黃素T(ThT)測定和透射電子顯微鏡(TEM)觀測纖維化的影響。這些化合物之一,diCA,其結構是以約5.0 Å長度的丙二胺連接二個以醯胺形式存在的咖啡酸(CA,此長度接近於Aβ纖維中分子間β片的距離)顯示出良好的抑制Aβ纖維化的能力,並且減少Aβ在人類神經母細胞瘤和Aβ轉殖線蟲中的毒性。此外,透過使用電噴灑離子化 – 行波離子遷移率 – 質譜(ESI-TWIM-MS)我們證明了diCA與Aβ的專一性結合,並且由分子模擬我們獲得Aβ和diCA複合物的細部結構。我們的成果提出了一個新的AD治療潛在策略。