以hTERT基因探討DNA甲基化與G-quadruplex之交互影響基因表現機制
Investigate the biological effect of DNA methylation on G-quadruplex of hTERT gene
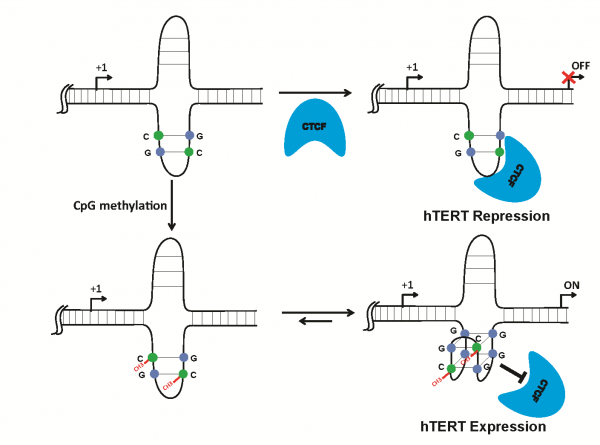
DNA甲基化(methylation)修飾為重要的表觀遺傳(epigenectic modification)調控方式,特別是啟動子(promoter)區域受DNA甲基化調控與癌症發展有極高的關聯性。DNA上的non-B二級結構如G-quadruplex被認為具會影響基因的表現的可能。但G-quadruplex結構形成序列上存在甲基化修飾對DNA結構及基因表現調控的可能影響,則仍不清楚。細胞癌化的重要特徵之一為其具端粒酶(telomerase)的活性,人類端粒酶反轉錄酶hTERT (human telomerase reverse transcriptase)作為端粒酶的主要組成之一,其基因過度表現對於端粒酶活性的影響扮演主要角色。文獻中已知hTERT 轉錄調控與DNA甲基化的位置有關。第一個外顯子(exon) 之高度甲機化(hypermethylation)可阻止轉錄阻遏因子(repressor) CTCF (CCCTC-binding factor)結合,為大部分癌細胞之hTERT基因表現所需。以基因序列分析,該CTCF結合區域也存在具有G-quadruplex結構的可能性,故合適以之探討G-quadruplex和DNA甲基化兩者可能的交互作用及影響轉錄因子結合調控基因表現機制。本研究以核磁共振(Nuclear Magnetic Resonance, NMR)確認受到甲基化調控的CTCF結合位序列會形成G-quadruplex結構。我們建構了含hTERT 啟動子區域且包含不同甲基化修飾之報導基因質體(reporter gene plasmids),從報導基因表現結果證實位於CTCF結合位之G-quadruplex結構,會和hTERT甲基化調控有交互影響而改變基因表現。也利用染色質免疫沉澱(Chromatin Immunoprecipitation)及凝膠遷移滯後實驗(electrophoretic mobility shift assays)確認CTCF結合能力會因為此交互作用影響而改變。本研究證明了G-quadruplex結構和甲基化之間的互相影響會透過影響轉錄因子CTCF的結合而改變hTERT基因表現,此為一過去未被報導過的hTERT基因調控分子機制。
DNA methylation is one of important epigenetic hallmark that regulating gene expression, especially for methylated CpG dinucleotide wildly spread in proximal promoter region leads to reverse gene activation between cancer and normal cell. G-quadruplex structure is a non-B DNA secondary structure formed in G-rich regions and involves in regulating gene expression. However, it remains unclear whether the status of cytosine methylation in the G-quadruplex will affect the DNA structure and further modulate gene expression. Cell immortalization is a key event in carcinogenesis, which requires activation of telomerase. Human telomerase reverse transcriptase (hTERT) is the major component of the catalytic subunit of telomerase and over-expressed in the majority of cancers. It has been shown that CTCF binding to the proximal exonic region of the hTERT gene could suppress its transcription in telomerase-negative cells, while the methylation of the first exon of hTERT prevents CTCF binding and results in hTERT gene expression in telomerase-positive cells. The CTCF binding site of hTERT first exon is capable of forming G-quadruplex structure via sequence analysis as well. In this project, we found that the methylation in the CpG islands could stabilize the G-quadruplex structure via NMR analysis. By results of reporter assays with different methylation pattern and mutant reporter plasmids, we found that the methylation promoted G-quadruplex structures in this CTCF binding region could alter the binding of CTCF and following hTERT expression. The results of ChIP and EMSA were also demonstrated that the relationship between CTCF binding and DNA methylation within G-quardruplex. Our results not only provide the possible explanation for transcriptional activation of hTERT but also offer a new example of how CpG dinucleotide methylation modulated DNA secondary structural formation.