結合計算與實驗策略探索線蟲個體間發育之異質性
Exploring Developmental Heterogeneity in C. elegans using both computational and experimental strategies
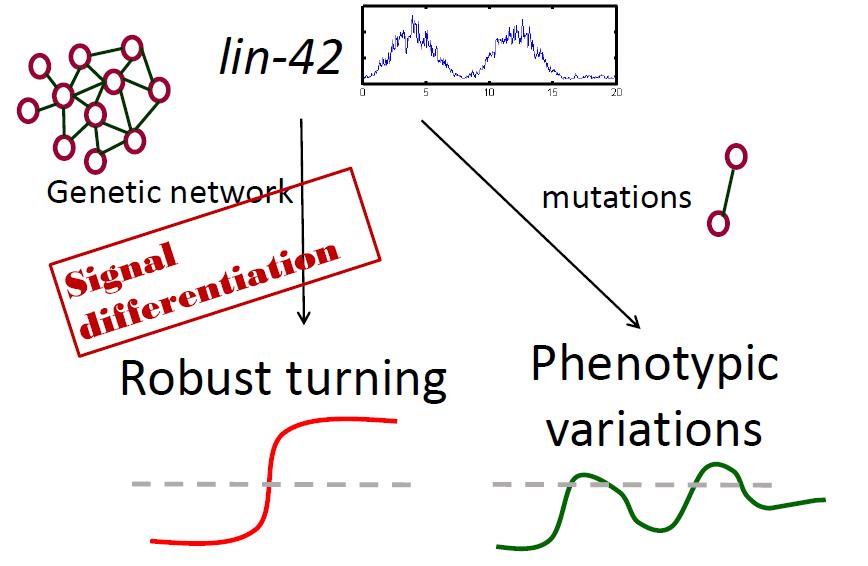
During animal development, spatial and temporal signals are integrated to generate elaborate patterns of cell migration. Compared to spatial signals, temporal regulation is relatively unexplored, and the mechanisms integrating both temporal and spatial signals are largely unknown. Despite of much qualitative and descriptive information is known for cell migration, very few quantitative or predictive models have been built to describe the dynamic pattern of cell migration during development. We use C. elegans distal tip cell (DTC) to investigate the mechanism of temporal control of cell migration. Two distal tip cells (DTCs) in C. elegans hermaphrodites migrate with a specific pattern in development and guide the gonad development. Initially migrating along the length of the body, DTCs abruptly turn 90 degrees and move from ventral to dorsal during the 3rd larval stage. The timing of DTC dorsalward migration is controlled by the expression of unc-5 receptor to repulse the ventrally expressed guidance cue UNC-6. Here we proposed a genetic network to control the timing of unc-5 expression. Combing with Computational simulations and experimental approaches, we built a mathmatical model to understand the molecular mechasim behind tightly temporal control of cell migration in C. elegans. we found a molecular threhold to determine the dorsalward migration timing of dital tip cell (DTC) migration. The tightly dorsal migration is regulated by mutiple temporal inputs interlinked into a gene regulatory network. We decipher the functional roles of these mutiple temporal inputs and clarify the role of gene interactions to integrate these signals. We found a cyclical period input enhance the a gene expression function as the brake to inhibit precocious dorsalward turning, whileas a stage specific temporal input switch the brake role into a padal to accerlarate the dorsalward turning. Removal both of the brake and padal function leads to the temporal heteregeneity in mutants. Our work demonstrate how an cyclical temporal siganl is differentiated to robustly keep a stage-specfic cell migration response.
在動物發育的過程中,細胞遷徙的過程整合了空間與時間的訊號。相較於空間訊號的調控機制,時間訊號與空間及時間訊號互相調節機制的了解甚少。此外,儘管已有不少研究敘述性針對細胞遷徙過程的分子機制進行描述,利用數學模型來了解細胞遷移動態行為的研究仍十分有限。我們利用線蟲的 DTC 細胞對細胞遷徙的時間調控機制進行了解。線蟲 DTC 細胞遷徙的時程決定了腸道的發育型態。其中,unc-5 接受器的表現會使得 DTC 細胞接受位於腹部表現的 UNC-6 的驅使,使得 DTC 在幼蟲的第三個時期進行從腹部往背向的遷徙。在此研究中,我們利用實驗與數學建模的方式提出了一個調控 unc-5 接受器表現的基因調控網路,並建立了一個調控線蟲細胞遷徙時間的數學模型。我們發現了一個可用於決定細胞遷徙時間的分子閾值,此分子閾值的表現量受到一個整合了許多時間訊號的基因網路所調控。我們進而針對這些時間訊號與基因網路間的調控機制進行了解。我們發現一個週期性的時間訊號開啟了基因網絡的煞車功能,然而另一個在特定表現的時間訊號能將此煞車功能轉化成油門,進而加速 DTC 的遷移行為。基因突變同時失去了煞車與油門的功能將導致細胞遷徙表現形態的異質性。我們的研究提出區分週期性訊號的分子調控機制,此機制將可應用於其他受到週期性訊號調控的細胞,使其在特定時間產生發育行為。