藉CLEC5a及共生微生物群調控先天免疫記憶
Regulation of innate immune memory by CLEC5a and microbiota
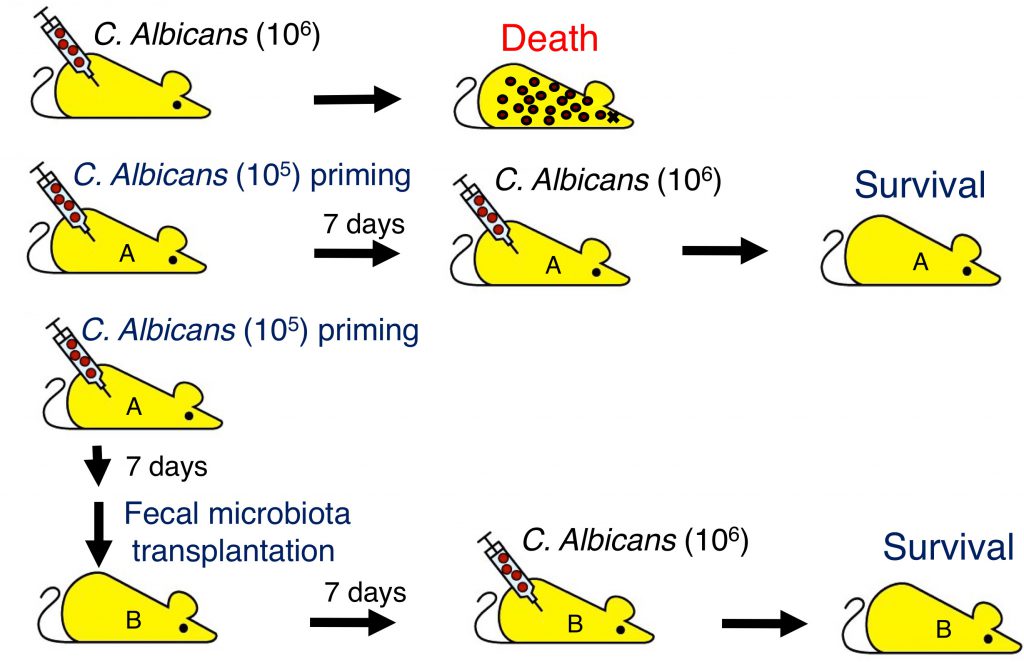
Innate immune memory is a newly identified immune machinery to generate trained innate immunity toward microbial infection, yet its molecular mechanism and regulation remain incompletely understood. In this study, we have first demonstrated that priming with low dose of Candida albicans protected host from subsequent lethal infection, indicating the generation of innate immune memory which is likely C-type lectin-dependent. We further demonstrated that protective innate immune memory mediated by low dose C. albicans priming could be transferred to naïve mice by fecal microbiota from primed mice. Recipients of such fecal microbiota transplantation became resistant to lethal C. albicans infection. This is the first demonstration that priming of non-lethal doses of pathogen conditions intestinal microbiota, and those microbiota contribute to the generation of innate immune memory. We also conducted extensive microbiome analysis, and identified bacteria that are specifically induced by C. albicans priming including Bacateroides, Parabacteroides, Mucispirillum, Dehalobacterium, Oscillospira, Ruminococcus, and Sutterella. Our results reveal a new mechanism on the generation of innate immune memory, and provide a new approach to modulate innate immune memory against pathogen infection and possibly control of auto-inflammatory or autoimmune diseases.
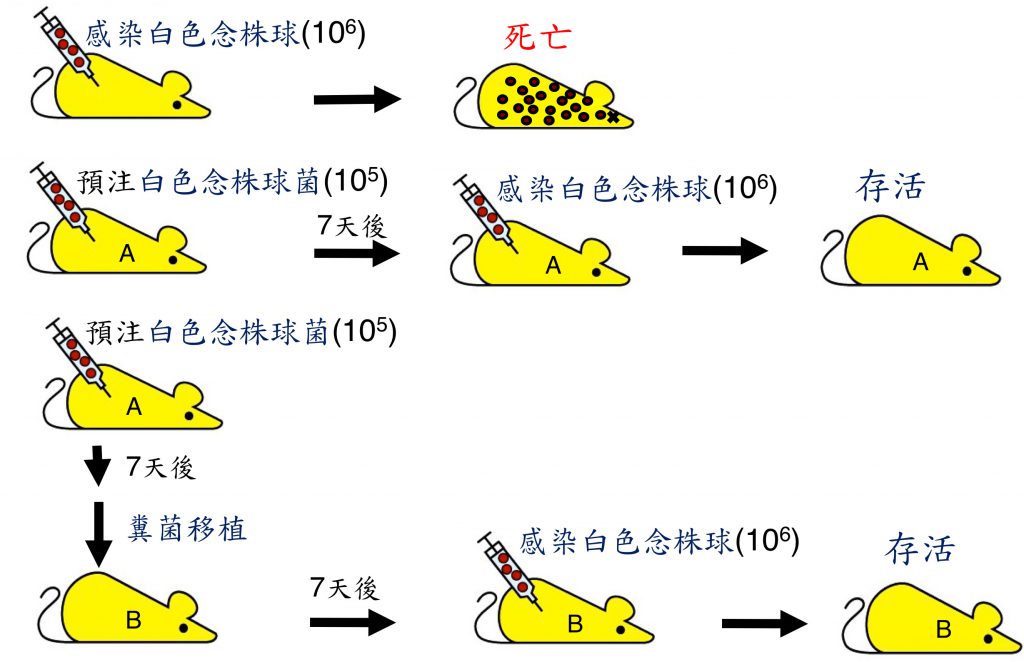
先天免疫記憶是最近發現的免疫機制可以訓練先天免疫用來對抗病原感染,然而其分子基礎及如何調控仍很不清楚。在本計畫中,我們首先重現小鼠打入低量白色念株球菌,可保護宿主抵抗其後致命性感染,顯示很可能是經由 C-type lectin 產生了先天免疫記憶。我們更進一步展示低劑量白色念株球菌所引發的保護性先天免疫記憶可經由糞菌移植轉移到樸真小鼠。接受如此糞菌移植的小鼠能抵抗致死劑量白色念株球菌感染。這是第一次顯示宿主預先打入低劑量病原會調理腸道菌落,而腸道菌參與先天免疫記憶的產生。我們也進行了詳細腸道菌分析,找出低劑量白色念株球菌誘發增加腸菌,包括Bacateroides, Parabacteroides, Mucispirillum, Dehalobacterium, Oscillospira, Ruminococcus, 及 Sutterella。我們的結果揭示了產生先天免疫記憶的新機制,並提供一個新方式以調控先天免疫記憶來對抗病原感染,及可能用來控制自體發炎及自體免疫疾病。