環狀掌性三配位硼陽離子之合成與不對稱催化反應
Cyclic Chiral Borenium Cations: Synthesis and Asymmetric Catalysis
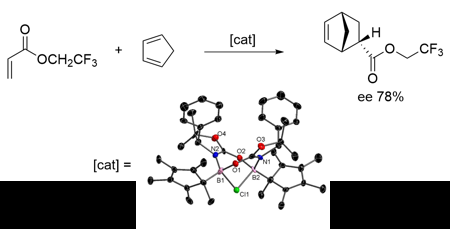
With the intrinsic high Lewis acidity of tri-coordinate boron cation, borenium cations have been intensively investigated in asymmetric catalysis. Compared with the well-documented mono-nuclear counterpart, cations featuring multiple boron atoms are rather limited and their catalytic application remains unexplored. Inspired by the excellent performance of the 1,1’-binaphthyl-derived diboranes in ketone hydrosilation and imine hydrogenation, we decided to extend the chemistry of chiral boron cation from the mono-nuclear system to the di-nuclear one. In this project, a series of C2 symmetric cyclic chiral diboron complexes (1) obtained from dimerization of oxazolidinone-functionalized chloroboranes were synthesized and structurally characterized. Application of these diboron complexes as the pre-catalysts of asymmetric Diels-Alder reaction was also examined. A series of low-temperature NMR studies suggested that the enantioselectivity was controlled by a C2 symmetric chloride-bridged diboron cation [2]+. Besides, we also discovered that the coordination of the bridging chloride anion with SnCl4 resulted in a more congested environment around the boron atoms, leading to a significant improvement in the enantioselectivity of the catalyst.
不對稱合成在製藥及精細化學工業有著相當重要的地位,目前工業上仍然以不對稱的過渡金屬催化劑的應用最為廣用。然而,為了解決過渡金屬的地殼含量匱乏及其生物毒性,強路易士酸性的三配位硼陽離子也開始被應用在不對稱催化上。相較於單核硼陽離子,具複數硼原子的陽離子的文獻相對少,且從來沒有被用在催化反應上。在本研究,我們製備了一系列的具C2 對稱性的環狀掌性雙硼化合物 (1),此化合物由含有惡唑烷酮(oxazolidinone)的氯硼烷(chloroborane) 透過二聚化而形成。此雙硼化合物可作為不對稱Diels-Alder反應的催化劑前驅物。在一系列的低溫研究中顯示,此不對稱催化的選擇性是由一個具有氯離子架橋的環狀雙硼陽離子 [2]+ 中惡唑烷酮上的取代機立體障礙控制。此外,我們還發現了當加入另外一當量的四氯化錫(SnCl4)可以讓 [2]+ 有更高的立體選擇性,這是因為架橋氯離子會再與四氯化錫配位,進而在硼原子周圍創造更加擁擠的環境,造就了更高的立體選擇性。