TDP-43蛋白片段寡聚物之生化及生醫研究
Oligomeric Aggregates from TDP-43 Fragments for Biochemical and Biomedical Studies
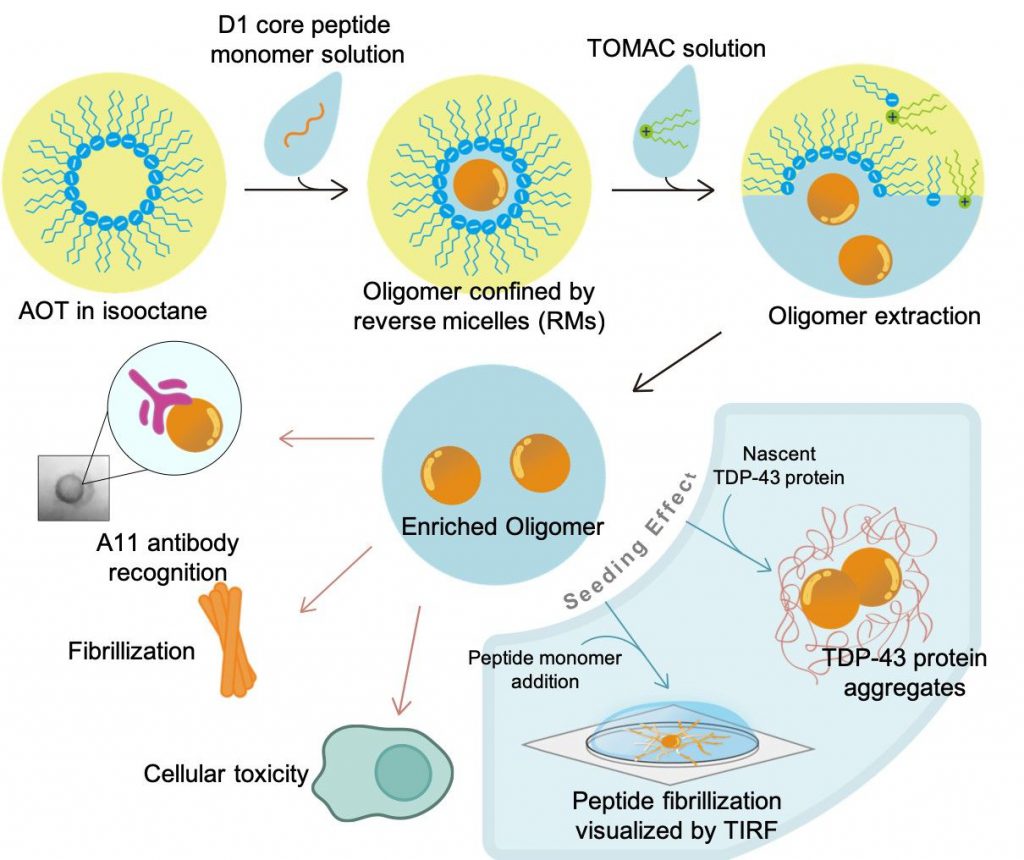
Amyloidogenesis in tissues or organs are closely related to different neurodegenerative diseases, including Alzheimer’s disease, Huntington’s disease, and Parkinson’s disease. Pathogenic amyloids form fibrillar plaques depositing in cells. Rather than fibrils, recent evidences have suggested the soluble intermediates during the protein aggregation process, such as oligomers, were relatively toxic and responsible for pathogenesis. Unfortunately, the oligomeric intermediates were unstable and short-lived, which makes the purification process and the following observation become more difficult. In this study, we aim to establish a new strategy to stabilize the “TDP-43 oligomers” and prevent them from transforming into fibrillar states. To achieve this goal, reversed micelles (RMs) were applied to encapsulate amyloid peptides. The confined space of central aqueous phase was able to contain the integrity of oligomers and prevent them from further fibrillization. We showcased this strategy was able to enrich oligomers derived from TDP-43 amyloid sequence, D1 core peptide. By extracting the oligomers from RMs, different physiochemical properties (e.g. toxicity and seeding effects) of D1 core could be probed by various tools, including western blotting and fluorescence microscopy.
The oligomeric state of freshly extracted D1 core was confirmed by oligomer-specific antibody, A11, comparing to either the monomeric or fibrillar D1 core. From the transmission electron microscopy (TEM) images, the D1 core oligomers were identified as spheres with a diameter around 25 nm. We had also shown that these oligomers can induce the TDP-43 protein aggregation in the cell-free system. Furthermore, the oligomers sedimented on glass surface seeded the newly added monomeric peptides into fibrils. Finally, we characterize the cellular toxicity of D1 core oligomers in U2OS cell lines and shown it stronger toxicity comparing to monomers or fibrils.
許多神經退化性疾病‚如阿茲海默症、亨丁頓氏舞蹈症、或帕金森氏症,都被發現和蛋白質或胜肽的類澱粉狀沉積有關,而不同疾病中,和病理相關的纖維狀的類澱粉沉積,也常被發現出現在不同細胞或組織中。然而,越來越多的證據指出,其實造成毒性或致病的關鍵並非最後觀察到的纖維狀沉積,而是聚積過程中出現的寡聚物。不幸的是,這些寡聚物很不穩定且生命期較短,因此不容易被純化及研究。為了解決這個問題,我們希望能以逆相微胞來穩定這些寡聚物,在逆相微胞中心的水相,因為有限的空間,寡聚物無法形成體積更大的纖維沉澱。利用逆相微胞,我們成功將TDP-43衍生的澱粉樣胜肽D1 core包覆,並在萃取後拿到高純度的寡聚物,而此寡聚物的性質(如:毒性、引晶效應)也能透過簡單的生物測定實驗來探討,像是西方點墨法或螢光顯微鏡。
利用對寡聚物有專一性的抗體(A11),我們證實了逆相微胞萃取的寡聚物,相較於單體或纖維狀沉積有很強的訊號。也在穿透式電子顯微鏡中,觀察到直徑約25奈米的球形寡聚物。接著,我們將寡聚物加入表達TDP-43蛋白的無細胞系統中,發現寡聚物有很強的引晶效應;另一方面,我們將寡聚物沉積在玻璃上,並用螢光顯微鏡直接觀察新加入的單體以寡聚物沉積點為中心向外沉積、生長成纖維狀堆積。我們同時利用U2OS細胞株,研究寡聚物的毒性,比起單體或纖維狀沉積,寡聚物造成了約一半的細胞死亡。